Recent years have witnessed remarkable breakthroughs in gene therapy. To date, more than 225 AAV-based clinical trials have been initiated worldwide, and six AAV gene-therapy products have received FDA approval for indications including hemophilia, inherited blindness, and spinal muscular atrophy. Adeno-associated virus (AAV) vectors have become one of the most frequently used delivery platforms because of their non-integrating nature and inherent tissue tropism. Nevertheless, translating AAV research from laboratory scale to clinical manufacturing still faces critical upstream-production and downstream-purification challenges that must be solved to guarantee sufficient vector supply.
01. AAV Production and Purification Challenges
The manufacturing workflow for recombinant AAV (rAAV) can be divided into three consecutive sections: upstream processing, downstream processing, and final formulation/fill-finish.
- Upstream processing: generation of viral particles in producer cells.
- Downstream processing: capture and polishing steps aimed at removing product- and process-related impurities to achieve the target vector purity.
- Formulation / fill-finish: development of a stable drug-product composition and its aseptic filling into final containers.
Because crude harvests must always be further purified, downstream operations represent a major proportion of total production costs and technical complexity. Consequently, robust and scalable purification strategies that deliver high-purity vector are essential.
Typical rAAV downstream platforms currently comprise:
- Cell lysis
- Nucleic-acid removal
- Clarification (removal of cell debris)
- Affinity chromatography (depletion of host-cell proteins, HCPs, and serum proteins)
- Density-gradient ultracentrifugation or ion-exchange polishing to separate DNA-containing, infectious full capsids from empty, non-infectious particles
- Final sterile filtration and formulation
After cell lysis, large amounts of host-cell DNA, RNA, and other nucleic-acid fragments are released. These contaminants reduce vector purity and can compromise product safety and efficacy. To mitigate this risk, broad-spectrum nucleases—most notably Benzonase—are routinely introduced into the process.
02. Application of Broad-Spectrum Nuclease in AAV Manufacture
Benzonase (a genetically engineered endonuclease from Serratia marcescens) cleaves phosphodiester bonds in all forms of DNA and RNA (single-stranded, double-stranded, linear, circular, or supercoiled), generating 5´-phosphorylated oligonucleotides of 2–5 bases in length.
According to WHO and major pharmacopoeial agencies, residual host-cell DNA in biologics must not exceed 100 pg per dose; in special cases the limit is 10 ng per dose. Large-scale AAV production implies massive cell expansion; consequently, lysates contain high levels of host-cell DNA and RNA. Incorporating Benzonase into the purification train efficiently reduces host-cell DNA (hcDNA) to levels well below regulatory thresholds, thereby minimizing the risk of dose-limiting impurities.
Optimal nuclease performance depends on several parameters: reaction temperature, buffer composition, incubation time, enzyme concentration, and the conditions used for enzyme removal or inactivation.
03. Product Highlights – Benzonase Broad-Spectrum Nuclease

- Exceptional stability
- Specific activity: ≥ 1,000 kU mg⁻¹ protein
- Animal-origin-free (AOF): eliminates the risk of adventitious agents
- Low endotoxin: manufactured in E. coli under GMP-compliant conditions; no detectable protease activity
- Secure supply chain: rapid, reliable delivery worldwide
[01]. Purity Data
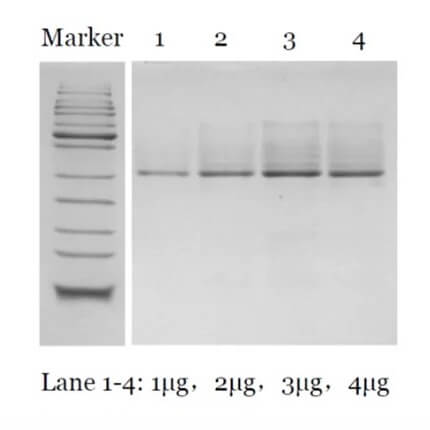
Purity ≥ 90 % (native-PAGE)
[02]. Protease Contamination Assay
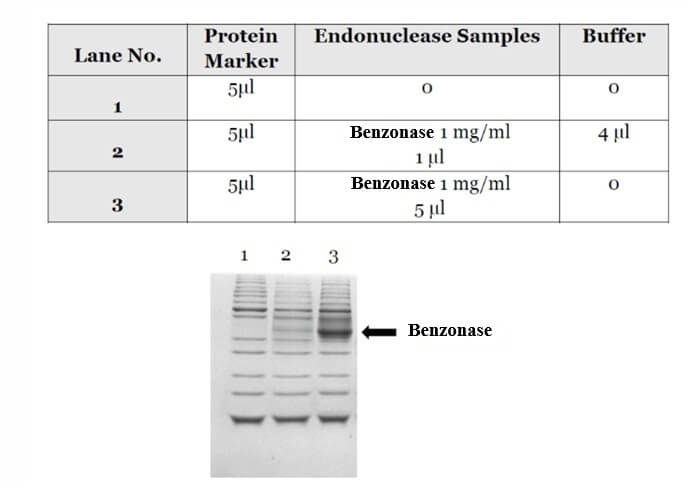
No protease activity detected
Ordering Information
| BE017 | Benzonase, GMP Grade |
| BE02/BE03 | Benzonase Lyophilized Powder |
Conclusion
Benzonase broad-spectrum nuclease is an indispensable tool for removing DNA and RNA contaminants during AAV vector production and purification. Proper implementation enhances vector purity, improves safety profiles, and delivers high-quality AAV drug substance for gene-therapy and biomedical research. Continued process innovation and cost optimization around nuclease-based clearance steps will further strengthen the commercial viability of AAV therapeutics.